Eurofins Advinus provides a one stop solution for biologics/ biosimilar testing which includes Toxicology, TK and Immunogenicity assessment. Eurofins Advinus has evaluated more than a dozen biologics/ biosimilar for toxicology studies.
Eurofins Advinus offers the following Biologics/Biosimilar services.
- Toxicology studies (GLP)
- Single dose Toxicity study in most relevant species (mice/rats/dogs)
- Repeat Dose Toxicity study with Recovery & Immunogenicity (mice/rats/dogs)
- Local Tolerance Study in rabbits
- Genotoxicity (GLP)—–For Novel vaccine adjuvants and additives
- Ames Test
- In vitro chromosomal aberration test in Human Peripheral Blood Lymphocytes (MUT-CHAB/HPBL)
- Micronucleus test (MNT) in mice/rats
- Safety Pharmacology (GLP)
- hERG assay
- Pulmonary functions (rats)
- Modified Irwin Test/Functional Observation Battery in rats (FOB-rats)
- CVS function assessment using telemetered dogs.
- Toxicology studies (GLP)
- Single dose Toxicity study in most relevant species (mice/rats/dogs)
- Repeat Dose Toxicity study with Recovery & Immunogenicity (mice/rats/dogs)
- Carcinogenicity Studies in rats or mice or Transgenic mice—For Novel vaccine adjuvants and additives
- Local Tolerance Study in rabbits
- Juvenile toxicity studies in rodents
- Humoral or Cell Mediated Immune (CMI) Response Assessmen
- Reproduction studies (GLP)
- Fertility and early embryonic development to implantation (SEG-I)
- Prenatal developmental study (SEG-II)
- Peri-natal and post-natal developmental study (SEG-III)
Biologics are complex entities produced by living systems and are used currently in various therapies. Their analysis is more complex and challenging than traditional small molecules. These biologics can generate immune responses that can affect its efficacy and also cause severe allergic and anaphylactic responses. Eurofins Advinus has wide experience in developing and validating sensitive and specific analytical/immunogenicity methods for all your biologics analysis needs. The methods are designed and optimized specifically to the requirements of your biologic product such as peptides, enzymes, monoclonal antibodies, vaccines, and gene therapy products. Our team is experienced in different modes of execution for biologics services: end-to-end development, validation and sample analysis; method transfer and validation; optimization of commercial kits and sample analysis etc.
- Generation of specific critical reagents in-house (polyclonal antibody)
- Assessment of pharmacokinetic parameters using accurate and sensitive methods validated as per latest guidelines
- Immunogenicity assessment by multi-tiered approach
- Anti-drug-antibody (ADA) screening assay
- Confirmatory assay
- Titre estimation
- Neutralizing antibody assay by ELISA or cell-based assays
- Antibody isotyping
- Functional bioassays
- Biomarker analysis such as Cytokines
- Well defined strategies for specific assays
| PK/ TK Assay | ADA Assay | |
| ELISA format | Sandwich ELISA preferred | Bridging ELISA preferred |
| Key Requirement | Good dynamic range to cover the Cmin and Cmax | High sensitivity to detect low binding ADA (at least 100 ng/mL) |
| Key Challenges | Demonstrate dilution linearity and parallelism | Detect ADA in presence of high levels of circulating drug concentrations and Drug–ADA complex |
List of available methods for different Biologics/Biosimilars
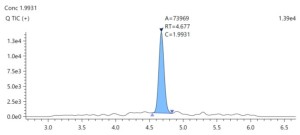 LC-MS method for quantification of Etanercept in plasma |
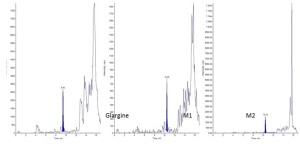 LC-MS method for quantification of Insulin Glargine in rat plasma |
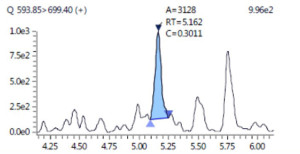 LC-MS method for quantification of Cetuximab in plasma |
|
Rituximab:
| ADA ELISA – Immunoassay for detection of antibodies to Rituximab in human serum | PK ELISA – Bioanalytical method for quantification of Rituximab in human serum | ||
|---|---|---|---|
| Parameters | Method Validation Results | Parameters | Method Validation Results |
| Sensitivity | 8 ng/mL | Linearity Range | 8 to 256 ng/mL |
| Precision | Global %CV of positive control (inter & intra) ≤ 20% | Dilution Linearity | 40,000 fold |
| Selectivity | 10 serum samples (6 normal, 2 lipemic, 2 hemolytic) met pre-defined acceptance criteria | Assay Range | Up to 256 ng/mL in serum samples |
| Robustness | Demonstrated across different analysts and over different sample incubation durations | Limit of Quantification | 8 ng/mL |
| Free Drug Tolerance | 25 ng/mL of Rituximab at 10 ng/mL of ADA | Accuracy | 87.9 to 99.82% (at all QC levels, n=25) |
| Precision | Global %CV ≤ 20% (at all QC levels, n=25) | ||
Bevacizumab:
| ADA ELISA – Immunoassay for detection of antibodies to Bevacizumab in human serum | PK ELISA – Bioanalytical method for quantification of Bevacizumab in human serum | ||
|---|---|---|---|
| Parameters | Method Validation Results | Parameters | Method Validation Results |
| Sensitivity | 187.5 ng/mL | Linearity Range | 60 to 2000 ng/mL |
| Precision | Global %CV of positive control from all precision batches (inter & intra) ≤ 20% | Dilution Linearity | 40,000 fold |
| Selectivity | 5 serum samples (3 normal, 1 lipemic, 1 hemolytic) met pre-defined acceptance criteria | Selectivity | 10 serum samples (6 normal, 2 lipemic, 2 hemolytic) met pre-defined acceptance criteria |
| Robustness | Demonstrated across different analysts and over different sample incubation durations | Assay Range | Up to 2000 ng/mL in human serum samples |
| Free Drug Tolerance | 3.125 ng/mL of Bevacizumab at 100 ng/mL of ADA | Limit of Quantification | 60 ng/mL |
| Accuracy | 83.41 to 113.25% (at all QC levels, n=25) | ||
| Precision | Global %CV ≤ 20% (at all QC levels, n=25) | ||
Aflibercept:
| PK ELISA – Bioanalytical method for quantification of Aflibercept in plasma | |
|---|---|
| Parameters | Method Validation Results |
| Linearity Range | 50 to 2000 ng/mL |
| Dilution Linearity | 8000 fold |
| Selectivity | 6 plasma samples met pre-defined acceptance criteria |
| Assay Range | Up to 2000 ng/mL |
| Limit of Quantification | 50 ng/mL |
| Accuracy | 76.44 to 104.99% (at all QC levels, n=12) |
| Precision | Global %CV ≤ 20% (at all QC levels, n=12) |
- Experience in various biologics/biosimilars such as Etanercept, Insulin Glargine, Rituximab, Bevacizumab, Trastuzumab, Aflibercept, Glatiramer acetate, etc.
- In vivo efficacy assays
- In vivo assessment of pharmacokinetic and pharmacodynamics parameters for biosimilar and comparison with innovator molecule
- In vitro pharmacological functional assays
- In vivo immunogenicity assessment for biosimilar and innovator molecule by multi-tiered approach-
- Anti-drug-antibody (ADA) screening assay
- Confirmatory assay
- Titer estimation
- Neutralizing antibody assay by ELISA or cell based assays
- Antibody isotyping
- Biomarker analysis
| PK/ TK Strategies | Immunogenicity Testing Strategies | |
| Type of ELISA | Sandwich ELISA preferred | Bridging ELISA preferred |
| Biosimilar comparability | Demonstration of equivalence with reference | Demonstration of equivalence with reference |
| Assay requirement | Assay with high sensitivity to cover the Cmin and Cmax | High sensitivity to detect very low levels of low affinity ADAs |
| Challenges with the Assay | Demonstrate dilution linearity and parallelism | Detect ADA in presence of high circulating drug concentrations and Drug–ADA complex |
- Ligand binding assays or Enzyme Linked Immunosorbent Assays (ELISA)
- Sandwich ELISA
- Bridging ELISA
- Direct/Indirect ELISA
- Competitive ELISA
- LC-MS/MS platform like API-6500+
- Immuno pull down, tryptic digestion and signature peptide identification approaches
- Cell-based assays using flow cytometry, fluorescence, luminescence readouts
- Critical Reagent generation and development
- Polyclonal antibody generation in rat, rabbit, guinea pig and chicken
- Protein A/G and Antigen specific purification
- Biotin, HRP and AP labelling
Anti-Drug Antibody (ADA) Development
- Polyclonal antibody generation
- In rat, rabbit, guinea pig and chicken
- Antigen: Biotherapeutic, full-length or truncated antibody, peptide
- Purification
- Protein A/G
- Antigen specific
- Labelling
- HRP, AP, Biotin and Digoxigenin
- ELISA development and validation for use in PK and Immunogenicity studies
Quantification of Biological Molecules by LC-MS/MS
- Assays can be developed and validated on LC-MS/MS platform like API-6500+
- Method development & optimization for the compound using immuno pull down, tryptic digestion and signature peptide identification approach
- Pre-study method validation
- Analysis of study samples for pharmacokinetics (PK), toxicokinetics (TK), pharmacodynamics (PD) and vaccine efficacy studies for quantification of proteins, peptides and monoclonal antibody based drugs
Case Study 1: Bioanalysis and Immunogenicity assessment for a novel biologic
CHALLENGE:
- Rat and NHP toxicity studies required GLP ELISA methods for bioanalysis and ADA assessment. However, there were no specific methods or reagents available for the test item (a novel biologic). Commercially available generic kits were tested at Eurofins Advinus. However, these kits did not recognize the test item probably due to differences in post-translational modifications.
SOLUTION:
- Polyclonal antibodies were generated in rat, rabbit and guinea pig using the test item. Antibody titers were checked at regular intervals from these animals. After reaching sufficiently high titers antibodies were purified and characterized.
- Bioanalytical methods were optimized using rabbit and guinea pig antibodies as capture and detection reagents. Anti-specific rabbit antibody was used as positive control for ADA methods. All methods were accurate, precise and sensitive.
- After completion of rat and NHP toxicity studies, the same method was successfully applied for PK samples from clinical study.
Case Study 2: Clinical Immunogenicity assessment for a novel biologic
CHALLENGE:
- GLP ELISA methods for ADA assessment in rat and mice serum had already been validated by Eurofins Advinus. However, when the method was applied in human serum, significantly higher background was observed. The signal/noise ratios were poor which required further troubleshooting.
SOLUTION:
- Several blocking reagents and conditions were tried to lower the background observed in human serum. PC concentrations and MRD were also further optimized without compromising the sensitivity of the method.
- ADA method was successfully reoptimized and validated. It was precise, selective and sensitive. Thereafter, it was used to analyse ADA samples from clinical study.
Case Study 3: Isotyping characterization of ADA during Immunogenicity assessment
CHALLENGE:
- Immunogenicity assessment for a peptide required in-depth characterization of the detected ADA including titer and isotyping. This method required generation of 5 different isotype specific ADA controls by crosslinking rabbit positive control antibody to various isotype controls.
SOLUTION:
- Several crosslinking conditions were tried to generate these conjugates. They were then checked for correct size and binding specificities.
- 5 Isotyping ADA methods were successfully developed and validated. These methods were precise, selective and sensitive. Thereafter, they were used to characterize isotypes of ADA positive samples.
Case Study 4: ADA method (Affinity Capture Elution) with high free drug tolerance for therapeutic monoclonal antibody
CHALLENGE:
- The biosimilar therapeutic monoclonal antibody had extremely high circulating levels in animal serum due to high dosage required for toxicity assessment. This necessitated an extremely free drug tolerant ADA assay which is not acheievable by typical acid dissociation techniques.
SOLUTION:
- Various methodologies were tried to achieve high drug tolerance. ACE method (Affinity Capture Elution) was successfully developed and validated. This method was highly drug tolerant, precise, selective and sensitive.
