Eurofins Advinus offers a wide range of DMPK services to support lead optimization and preclinical development. Eurofins Advinus capabilities and track record include:
- Highly qualified scientists trained in premier universities in India and abroad
- Performed over 500 PK studies, 5000 in vitro studies; supported over 200 Toxicology studies for regulated bioanalysis and toxicokinetics
- Bioanalytical team has developed over 120 validated methods for NCEs, supported over 90+ complete IND packages
A list of the assays and studies that can be performed is provided below.
in vitro potassium release assay for liposomal formulations of Amphotericin B
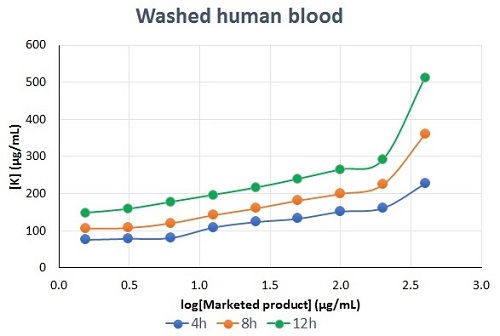
- Assay performed with blood from preclinical species or with human blood
- Achieved dose- and time-dependent potassium release from red blood cells
- Assay validated for determination of potassium concentration using ICP-MS platform
- Assay conducted in compliance with GLP and in non-GLP format
Physicochemical Properties
- Aqueous solubility and stability at different pH condition
- Stability in simulated gastric and intestinal fluid
- Octanol water partitioning (Log P and Log D)
- pKa determination
Permeability
- PAMPA
- Caco-2 cell line
- MDCKII cell line
P-gp Substrate and Inhibition Potential
- Caco-2 cell line
- MDCKII-P-gp transfected cell line
Metabolic Stability
- Stability in S9, microsomes and hepatocytes to assess potential for intestinal first pass, intrinsic clearance to evaluate in vitro clearance
Plasma Protein Binding
- Equilibrium dialysis and ultrafiltration techniques
- Blood/Plasma Partitioning and Stability
Tissue Homogenate Binding
CYP Reaction Phenotyping
- Using recombinant human CYPs as well as human liver microsomes
- Aldehyde oxidase reaction phenotyping
Metabolite Identification
- Using unlabeled and radiolabeled compound coupled with mass spectrometric detection
- Preliminary metabolite identification
- In in vitro and in vivo study samples
- Reactive metabolite trapping and characterization
Drug-Drug Interaction Assays
- CYP Inhibition using human liver microsomes (IC50, Ki determination)
- Time dependent CYP inhibition (IC50shift)
- CYP induction in human hepatocytes
- P-gp substrate and inhibition assays using Caco2 and MDCKII-P-gp cell lines
Cytotoxicity
- MTT Assay
- Species (rodent and non-rodent)
- Absolute and relative bioavailability assessment
- Cannulation techniques used: Jugular vein, femoral vein, carotid artery, portal vein and bile duct
- Dosing techniques: IV, Oral, IM, SC, intranasal instillation, endotracheal intubation, dermal application, intradermal etc.
- Brain penetration in vivo, supplemented with P-gp assessment in vitro
- Validated WinNonlin Phoenix software is used for PK data analysis
- Mass Balance / Excretion balance
- Tissue distribution
- Ocular PK study in rabbits
- Biliary excretion study
- Metabolite profiling
- Toxicokinetic data analysis and report preparation to support IND enabling studies
- Allometric scaling: FIH dose projections and human PK predictions
- Validated Phoenix WinNonlin® software
Ideally a lead compound should have > 30% oral bioavailability and < 30% liver blood flow clearance and an adequate half-life for once daily dosing. In the absence of this, to improve bioavailability, a suitable formulation needs to be developed. Eurofins Advinus has the capability to execute the following:
- Development of solution formulation for IV and PO studies of low solubility lipophilic compounds for conduct of preclinical PK studies using
- pH adjustment
- Co-solvents
- Inclusion complexes (cyclodextrins)
- Attempts are made to achieve formulation using excipients acceptable for use in repeated dose studies
- The prepared formulations are assessed for accuracy using HPLC or LC/MS/MS method
- If necessary, salt forms or pro-drugs can be considered for improving the bioavailability of lead compound along with micronization of compound
- Formulation method development and validation for preclinical dose formulation sample analysis
