IND Enabling Studies
Genotoxicity studies
- Ames test
- In vitro mammalian chromosomal aberration test in human peripheral blood lymphocytes (HPBL) and CHO cells
- In vitro mammalian cell gene mutation test in mouse lymphoma cells using the Tk gene and in CHO cells using the Hprt gene
- In vitro micronucleus test in human peripheral blood lymphocytes (HPBL) and CHO cells
- In vivo micronucleus test in rat and mouse
Safety Pharmacology In Vivo
- In vitro hERG assay (CHO/HEK)
- Pulmonary function in rats
- Modified Irwin test (FOB) in rats
- Cardiovascular telemetry in dogs
- Gastrointestinal motility in rats
Toxicology Studies (In Vivo)
- Maximum Tolerated Dose (MTD) toxicology studies in rodents (rats/mice)
- Maximum Tolerated Dose (MTD) toxicology studies in non-rodents
- Dose range finding toxicology studies in rodents (rats/mice)
- Dose range finding toxicology studies in non-rodents
- 28-day repeat dose toxicity studies in rodents (rats/mice) with toxicokinetics
- 28-day repeat dose toxicity studies in non-rodents with toxicokinetics
NDA Enabling Studies
Toxicology Studies (In Vivo)
- 90-day repeat dose toxicology studies in rodents (rats/mice) with toxicokinetics
- 90-day repeat dose toxicology studies in non-rodents with toxicokinetics
- 6 to 12 month repeat dose toxicology studies in rodents (rats/mice) with toxicokinetics
- 9- month repeat dose toxicology studies in non-rodents with toxicokinetics
- 2-year carcinogenicity studies in rats/mice
- 6-month carcinogenicity study in Transgenic rasH2 mice with toxicokinetics
- Fertility (SEG-I) studies in rats
- Teratogenicity (SEG-II) studies in rats/rabbits
- Perinatal and post-natal (SEG-III) studies in rats
- Juvenile toxicology studies in rats
- Standalone pathology studies
Impurity
Impurity Qualification Studies For Pharmaceuticals (In Vivo)
- Single dose toxicology studies in rodents (rats/mice)
- Single dose toxicology studies in non-rodents
- 14 to 28 day systemic toxicology studies in rodents (rats/mice)
- 14 to 28 day systemic toxicology studies in non-rodents
- 90 day systemic toxicology studies in rodents (rats/mice)
- 90 day systemic toxicology studies in non-rodents
- Standalone TK studies
Immunotoxicology (In Vivo)
- Guinea Pig Maximization Test
- Local Lymph Node Assay
Routes of Administration
- Oral through gavage, dietary or drinking water
- Inhalation
- Intra-peritoneal
- Intravenous slow bolus or intravenous infusion
- Intramuscular
- Intradermal
- Subcutaneous
- Ocular
- Dermal
Pathology Services
We now offer Stand Alone Pathology Services for the in-life phase of the studies conducted elsewhere.
Immunohistochemistry
- Immunohistochemistry (IHC) combines anatomical, immunological and biochemical techniques to identify discrete tissue components by the interaction of target antigens with specific antibodies tagged with a visible label.
- IHC makes it possible to visualize the distribution and localization of specific cellular components within cells and in the proper tissue context
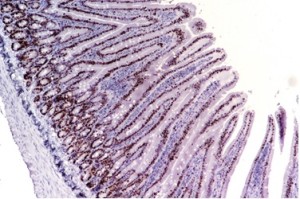 BrdU- IP route 50 mg/kg Intestine 10x |
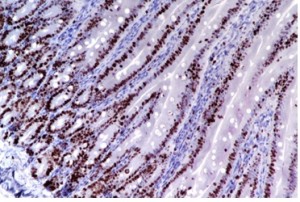 BrdU- IP route 50 mg/kg Intestine 20x |
Neuropathology
- To identify neurotoxic potential of new chemical entities in pharmaceutical industries
- To evaluate neurotoxicity potential of agro and environmental chemicals
- Brain morphometry procedures for the young adults and juvenile rats
- 100% harvest of the Dorsal root ganglion for microscopic examination
- Guidelines –
- OECD 424: Neurotoxicity Study in Rodents
- OECD 418: Delayed Neurotoxicity of Organophosphorus Substances Following Acute Exposure
- OECD 419: Delayed Neurotoxicity of Organophosphorus Substances: 28-day Repeated Dose Study
- OECD 426 and US EPA OPPTS 870.6300 : Developmental Neurotoxicity Study
- US EPA OPPTS 870.6200: Neurotoxicity Screening Battery
- US EPA OPPTS 870.8600: Developmental Neurotoxicity Screen
- FDA Redbook 2000: IV.C.10 Neurotoxicity Studies: Toxicological Principles for the Safety Assessment of Food Ingredients
Quantitative Bone Marrow Evaluation
- To assess toxicant-induced bone marrow effects of test articles
- In nonclinical toxicity studies, complete blood count data in conjunction with the histological examination of the bone marrow are recommended as the foundation for assessing the effect of test articles on the hematopoietic system
- Expertise to conduct the cytological evaluations. In some situations it may be necessary to further characterize effects on the different hematopoietic lineages by cytological evaluation of the bone marrow
EpiSkin® Test in vitro Skin Corrosion & Irritation
- in vitro Skin Irritation (OECD 439)
- in vitro Skin Corrosion (OECD 431)
What is SEND?
- Standard for Exchange of Nonclinical Data (SEND) is a new electronic formatting requirement for submission of study data contained in certain Investigational New Drug (IND), New Drug Application (NDA), Abbreviated NDA application (ANDA), and Biologic License Applications (BLA) to the US FDA.
- Nonclinical studies must now implement the Study Data Tabulation Model (SDTM), which is the electronic standard used for clinical regulatory submissions to the FDA.
- SEND, SDTM, and associated standards are developed by CDISC (Clinical Data Interchange Standards Consortium), a non-profit standards development organization consisting of representatives from pharma, biotech, CROs, vendors, consultants, and the FDA.
- On December 17, 2014, FDA published a final guidance on the use of standardized electronic formats (i.e., SEND and SDTM) for applicable nonclinical and clinical submissions. These electronic standards have become mandatory for all studies starting December 2016.
Importance of SEND
- SEND is being used by the FDA to analyze data in study submissions
- Rapid access to large amount of data, faster FDA review and therefore faster time to market
- Uses controlled terminologies
- Standardized formats
- Enhanced data review
- Eliminates data entry by agency
- Enables data warehousing, analysis and visualization
- Noncompliance can result in a refusal to file from the FDA and submission delays.
Timeline for Implementation of SEND by FDA for all Sponsors, CRO etc.
The final FDA guidance on standardized electronic formats, issued on December 17, 2014, has become binding (i.e., SEND and SDTM will be required) as follows:
- For applicable NDAs, ANDAs, and BLAs: Studies starting after December 17, 2016
- For applicable INDs: Studies starting after December 17, 2017
SEND Readiness at Eurofins Advinus
- Eurofins Advinus started its evaluation in 2012
- The first SEND dataset for an in-house study was generated in June 2013
- Since then all FDA submission NDA studies performed at Eurofins Advinus use SEND
- SEND data submission for 3 Carcinogenicity studies is on-going
Process of using SEND at Eurofins Advinus
Study Director
⇓
Processing data in SEND format
⇓
Study team/QAU verifies all the modules, data
⇓
Ready for Submission
Eurofins Advinus Ensures that data sets are…..
- Data sets are consistent with the Study Reports
- Validated and loadable into Nonclinical Information Management System (NIMS) without getting Refusal to File (RTF) from the FDA
- Reviewed by the scientists to enable accurate interpretation and timely response to any questions
Analytical Support
Eurofins Advinus’ capabilities include dose confirmation studies (which is a regulatory requirement) for animal toxicology, eco-toxicology and genetic toxicology studies such as:
- Analytical method development and validation for analysis of test item concentration in
- Gavage suspensions / solutions
- Dietary admixtures
- In vitro incubations
- Air inhalation samples
- Reconstituted/aquarium water
- Algal medium, etc.
- Testing stability and homogeneity of the test item in dose formulations
Bioanalytical support
Eurofins Advinus’. bioanalytical group specializes in quantitative LC-MS/MS method development, method validation and study sample analysis. Bioanalytical services are routinely provided for compounds in discovery, and in preclinical (toxicology) and clinical development. Fit-for-purpose bioanalytical methods are developed for discovery studies. GLP-compliant bioanalysis using fully validated methods developed are performed for studies conducted for regulatory submission.
Bioanalysis of small molecules
- Bioanalytical services to support GLP IND enabling study package for FDA, EMEA, MHRA and other global regulatory submissions, and also for other studies requiring analytical support.
- Excellent capability to develop and validate bioanalytical methods using state-of-the-art LC-MS/MS & HPLC instrumentation and achieve sensitivity up to picogram levels
- Developing fit-for-purpose methods for non-GLP bioanalysis
- Sample processing using precipitation, liquid-liquid extractions and solid-phase extractions
- Capability for derivatization approaches (e.g. for poorly ionizable compounds) including method development and validation
- Quantification of analyte / metabolite(s) in biological matrices such as plasma, serum and blood samples in compliance with latest regulatory requirements of USFDA, EMEA, etc.,
- Experience with developing methods for other matrices such as urine, feces, CSF, bile and various tissue homogenates
- GLP bioanalysis to support preclinical bioequivalence studies
- Experience with specialized formulations such as liposomes
- Equipped with Watson LIMS – 21 CFR Part 11 compliant Data Management System for data
Bioanalysis large molecules
- Capability for method development and validation to comply with GLP requirements
- Bioanalytical method feasibility, method development and validation including method transfer using ELISA methods
- Validation using commercially-available ELISA kits
- Capability for peptide and protein bioanalysis by ELISA methods
- Preclinical (non-GLP and GLP studies)
- Immunogenicity/antigen-antibody assays including neutralizing antibody assays
- Capability for biomarker assays
- Biomarker identification and quantification
Formulation Analysis – Pharma
- Formulation analysis service for preclinical dose formulation samples as per current regulatory requirements
- Strong capability to develop and validate methods (GLP) using HPLC in dose formulations
- Quick and reliable fit-for-purpose formulation analytical method to support analysis of non-GLP / exploratory study formulation samples
Eurofins Advinus has a long history of performing Good Laboratory Practice (GLP) compliant toxicology studies for regulatory filing. Eurofins Advinus’ facilities has been GLP accredited by BfR German Health Ministry since 1992, when it became the first laboratory in India to get GLP accreditation . In 1999, W&V Netherlands, and in 2005, Indian GLP authorities also accredited this facility. In addition, the facility is also AAALAC International certified since 2001. The facility was also the first GLP facility in India, inspected by USFDA in 2012.
We have conducted more than 25,000 regulatory studies for submissions to regulatory authorities globally including USFDA, EMA, MHRA, TGA, Health Canada and DCGI. Eurofins Advinus has capabilities for full range of safety assessment (toxicology) studies required by agrochemicals, chemicals (REACH), dyes, and other such industries.
Safety Assessment studies conducted are compliant with OECD GLP and the study guidelines prescribed by the international regulatory agencies including but not limited to USFDA, USEPA (OPPTS), OECD, EEC, JMAFF, WHO, DCGI, DBT and CIB of India.
Our broad spectrum of GLP and non-GLP service offerings, along with our experience of over 3 decades, ensures meeting the current global toxicology testing requirements. Eurofins Advinus’ experienced team enables study completion in the shortest possible time period such that regulatory submissions can be expedited.
All animal study protocols are reviewed and approved by Institutional Animal Ethics Committee (IAEC) which is similar to IACUC of AAALAC International.
Eurofins Advinus’ vivarium is one of the largest in South Asia with over 80 rooms, which meets global standards of maintenance, operation and is approved by the CPCSEA, Government of India, and accredited by AAALAC International.
Eurofins Advinus has completed 90+ full IND packages for global regulatory submissions. Further, the company routinely undertakes chronic toxicity, reproduction toxicity, juvenile toxicity and carcinogenicity studies. The company has experience of 65+ carcinogenicity studies till date (including both pharmaceutical and agrochemical compounds) mostly for submissions to the US and EU regulators. The company has an extensive historical control dataset in rats (Wistar and Sprague Dawley) and mice (Swiss Albino). In addition to regular carcinogenicity studies, the company has also successfully performed 6 month carcinogenicity study in Tg RasH2 transgenic mouse model, a service offering that is offered by only a few global nonclinical contract research organizations.
Eurofins Advinus takes pride in the fact that the toxicology team has 35 highly qualified study directors with an average of 15 years of industry and regulatory exposure. Eurofins Advinus also has 9 Diplomates of the American Board of Toxicology (DABT) certified toxicologists.
IND Development Programs
- Eurofins Advinus is engaged in integrated drug development starting from CMC, DMPK, Analytical to Safety Assessment including cGMP material for supporting early clinical development up to Phase II clinical trials.
- While predominantly ICH Guidance documents are followed for all toxicology studies to comply with global preclinical requirement, where applicable, detailed OECD and others guidelines, would be followed to provide more specifics to study details.
- Laboratories are fully equipped with the latest equipment which are validated, regularly calibrated and manned by adequately trained, experienced staff.
Experience in 2-Year Carcinogenicity Studies
- Over two decades of experience conducting carcinogenicity studies in accordance with OECD/USFDA guidelines
- Highly skilled study directors with 15-20 years of experience handling carcinogenicity studies in rodents
- Eurofins Advinus has the largest animal vivarium used for conducting carcinogenicity studies in India
- Completed 65+ studies with extensive historical control data both for Agro and Pharma compounds
- Experience in conducting carcinogenicity studies using data analyzed and protocols approved by the Carcinogenicity Assessment Committee (CAC) of USFDA
- Survival of animals included in the carcinogenicity studies is > 80 %
- Data successfully submitted to regulatory agencies around the globe
- Recently successfully completed and submitted report for a 2 year rat carcinogenicity with BID dosing for a pharma compound
- Capability to compile study data files in accordance to FDA approved “Standard for Exchange of Non-Clinical Data (SEND)” format for regulatory submission
- Peer review pathology services by independent overseas ‘Board Certified Pathologists’ is offered
Experience in Carcinogenicity Studies Using Transgenic Mice
- Screening models for carcinogenicity assay using Tg rasH2 mice
- One of the few contract research labs world-wide to offer carcinogenicity study in Tg rasH2 transgenic mice
- Experience in conducting the study using data analyzed and protocols approved by the carcinogenicity assessment committee (CAC) of USFDA
- Survivability of 100% achieved in the vehicle control group in 6-month carcinogenicity study and data successfully submitted to USFDA
- Capability to compile study data files in accordance to USFDA approved “Standard for Exchange of Non-Clinical Data (SEND)” format for regulatory submission
Impurity Qualification Toxicology Studies
- Eurofins Advinus has excellent knowledge and expertise in assessing toxicity of impurity(ies) of pharmaceuticals in rodents as per ICH Q3A and ICH Q3B guidelines.
- Capability to test APIs with more than 1 up to 10 impurities.
- Eurofins Advinus has successfully designed and conducted more than 25 impurity qualification studies in rodents which have been accepted by various regulatory authorities around the globe including USFDA.
Pathology Services
We have an outstanding team of pathologists with over 30 years of pathology services across both pharmaceuticals and agrochemicals. Our work has been successfully peer-reviewed multiple times by board certified pathologists from US, Europe and Japan. Included in our team is Prof. Vijayasarthi, an IATP fellow and an expert pathologist with 35years of experience.
We now offer Stand Alone Pathology Services for the in-life phase of the studies conducted elsewhere. The formalin preserved/ wet tissues or paraffin embedded tissue blocks supplied by the client can be processed at our facility, the tissue slides evaluated and a comprehensive pathology report generated for submission through online data capture system.
Intravenous Infusion Studies
- With experts in intravenous infusion through tail vein in rats, Eurofins Advinus has conducted toxicological studies for evaluating parenteral drug formulations
- Capability is also available for continuous infusion up to 2 hours in rats. Eurofins Advinus has successfully completed 5 cycle IV infusion study and also 28-days repeat IV infusion study in rats to assess toxic effects of parenteral drug formulations
Local Lymph Node Assay (LLNA)
- Eurofins Advinus has more than a decade of experience in conducting LLNA studies in mice which has largely superseded the guinea pig maximization test and the Buehler test.
- Well-trained and experienced study directors are available to conduct LLNA studies in mice according to OECD guideline No. 429 to provide quantitative data suitable for dose response assessment
Juvenile Toxicology Studies
- Current regulatory environment for pediatric drugs is driving juvenile toxicology studies which are useful in addressing specific concern in pediatric trials.
- Eurofins Advinus has expertise in conducting juvenile rat toxicology studies and the reports have been submitted to various regulatory agencies including USFDA.
EpiSkin® Test - in vitro Skin Irritation & in vitro Skin Corrosion
Eurofins Advinus has broadened its scope of services through the introduction of in vitro Skin Irritation (OECD 439) and in vitro Skin Corrosion tests (OECD 431). These in vitro tests will be assessed using the Human 3-Dimensional EpiSkin® test kit. The tests have been validated in our laboratory by employing the OECD Guideline mandated proficiency substances. The in vitro EpiSkin® tests are routinely used for cosmetic raw materials and cosmetic formulations. In addition, current OECD guidelines for in vivo (whole animal) skin irritation tests mandate that prior to testing in animals all agrochemical and industrial (REACH) chemicals must be evaluated by employing in vitro tests. These tests are offered both under GLP for regulatory submission and also under non-GLP for screening in the research mode. Both in vitro EpiSkin® tests can be taken up in one week following supply of the compound and results can be provided with a week.
Electronic Data Processing & Historical Data Bank
- A decade long experience in data capture and protection: Provantis® is being used in all Toxicology/Clinical Pathology/Pathology areas.
- Computers networked with large number of workstations to enable on-line transfer of data
- Digital Pathology services
- Efficient and validated in-house software packages are used for statistical analysis and interpretation of data
- Historical data acquired over the years are available for clinical signs, body weight, feed intake, hematology and clinical chemistry, histopathology and tumor incidence. In addition, the historical data available for all types of reproduction studies, including fertility, pre and/ or postnatal studies.
Experience in BID dosing
- Drugs that are shorter half-life or rapid clearance or limited solubility and based on its clinical regimen, twice (BID) daily administration is generally preferred to maximize/achieve desired systemic exposure in nonclinical studies.
- Eurofins Advinus has the experience and capabilities of conducting 28-day and longer duration term toxicology studies with more than once daily administration, including carcinogenicity/reproduction studies in Rodents.
- Conducted 25+ repeated dose, reproduction and carcinogenicity studies, including carcinogenicity study in rasH2 Transgenic mice
- Over 120 Scientists with 65% with either a Ph.D., M.S. (Biology) or D.V.M degree
- 35 Study Directors with DABT/ERT Certified Study Directors
- Average experience of a Study Director –15 years
- 8 Veterinary Pathologists with DVM, MS with 5 to 10 years of Industry experience led by a pathologist with 30+ years of experience
- 17 Trained Histologists
- Studies peer reviewed and approved by Board Certified Pathologists from US, Europe and Israel
