Eurofins BioPharma Product Testing holds a proven track record of providing quality GMP testing services for the reknowned pharmaceutical and biopharmaceutical companies in the world. Eurofins BioPharma Product Testing is a global leader in bio/pharma laboratory services providing comprehensive, innovative, and timely solutions.
From Starting Materials through to Finished Product Testing, Eurofins BioPharma Product Testing’s network of over 45 facilities in more than 20 countries delivers the world’s most comprehensive scope of harmonized GMP testing services and seamless regulatory acceptance.
Eurofins BioPharma Product Testing, India, a cGMP-compliant and US-FDA inspected facility provides end to end analytical testing services to the pharmaceutical industries.
The state of the art testing facility in Bengaluru provides cGMP analytical testingservices which include method development/validation, raw material, intermediate and finished product release testing.
Services offered
The BPT analytical team is competent in handling various chromatographic and spectroscopic techniques.
Current services offered by Eurofins BPT, India are for Drug Substance, Drug Product, Intermediates and Excipients
- Elemental impurities evaluation as per ICH Q3D
- Genotoxic impurities evaluation as per ICH M7
- Extractable & Leachable studies for packaging, container closure system and medical devices
- Residual Solvents testing at trace level using GC Head Space technology with MS
- Nitrosamine impurities evaluation in drug substance and drug product per EU and USP regulation
- HPLC/GC method development, feasibility, verification, validation, and transfer.
- Forced degradation studies
- Release Testing
- Drug Substance /Drug product analysis as per monograph
- Raw material and excipients analysis
- Dissolution Profile studies
- Packing material analysis
- Photostability studies
- ICH and VICH stability studies
- Microbiology testing
Equipment
- High Performance Liquid Chromatography (HPLC) equipped with diode array, variable wavelength, and refractive index detector
- Gas Chromatography with Headspace using FID Detector
- Liquid chromatographic-mass spectrometry (LC-MS/MS)
- Inductively coupled plasma-mass-spectrometry (ICP-MS)
- Gas Chromatography Mass spectrometry (GC-MS/MS)
- Particle size analyser – Malvern Mastersizer 3000
- pXRD
- Ion Chromatography
- UV and FTIR spectroscopy
- Dissolution tester Type I and Type II
- Walk-In Stability chambers
Quality & Compliance
Eurofins Biopharma Product Testing India PVT LTD believes that meeting compliance obligations is a responsibility essential to its long-term success. Ensure the continued development, implementation, and maintenance of the world class quality system and to continually seek improvements in its effectiveness .
Quality Management system consisting of 4 layers
- Quality Manual
- Management System Procedures
- SOPs, Policy, Methods, Standards
- Formats
Quality System
- Organogram & Job Responsibilities
- Training and analyst qualification program
- Quality Manual
- Handling of deviations
- Handling of Out of Specification Results
- Handling of CAPA
- Change Control management
- Life Cycle Management of Equipment/Instruments
- Instrument Qualifications/ Calibrations
- Validation Master Plan
- Sample testing and release
- Computerized system validation, electronic data control and backup
Laboratory Data
The following data are captured online meeting 21CFR Part 11:
- Sample receipt, storage, and tracking
- Chemical’s inventory
- Analytical methods
- Sample preparation
- Instrument logs
- Temperature logs
- Reference standard inventory and use log
- HPLC and GC column inventory log and use log
- Analysis raw data
- Reports
- Secure, virus-free system
- 21 CFR Part 11compliant Software
- Electronic data backup and restoration
- Secure, virus-free system
- Archival Management
Any Time Audit Readiness
We are audit ready all the time through For Virtual or Onsite audit where you can review your samples journey in EBPTL at various stages. Virtual Audit enables you to cut your travel consequently saving your valuable time and cost of audit. We are equally pleased to welcome you in our facility at predetermined schedules. You can have more frequent audits depending on complexity of your project. We will be pleased to assist you in all aspects.
You are welcome to visit us in person too!
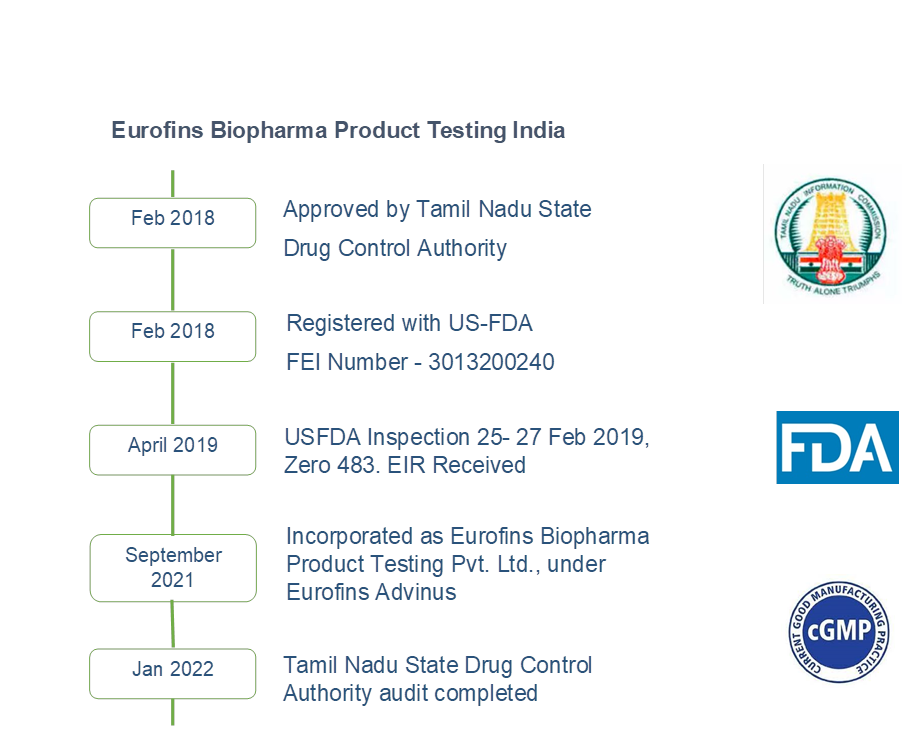
Journey of Eurofins BPT, India
Details of Validation
| Category | Type of Validation | Technique |
|---|---|---|
| Lidocaine HCl | Nitrosamines Testing | LCMS/MS |
| Lidocaine base | Nitrosamines Testing | LCMS/MS |
| Meloxicam | Nitrosamines Testing | LCMS/MS |
| Melatonin | Nitrosamines Testing | LCMS/MS |
| Pentosan polysulfate sodium | Nitrosamines Testing | LCMS/MS |
| Paracetamol | Nitrosamines Testing | LCMS/MS |
| Glipizide | Nitrosamines Testing | LCMS/MS |
| Glibenclamide | Nitrosamines Testing | LCMS/MS |
| Furosemide | Nitrosamines Testing | LCMS/MS |
| Zileuton | Nitrosamines Testing | LCMS/MS |
| Pentoxifylline | Nitrosamines Testing | LCMS/MS |
| Sapropterin dihydrochloride | Nitrosamines Testing | LCMS/MS |
| Paroxetine Hydrochloride Hemihydrate | Nitrosamines Testing | LCMS/MS |
| Diphenhydramine Citrate | Nitrosamines Testing | LCMS/MS |
| Methohexital API | Nitrosamines Testing | LCMS/MS |
| Prilocaine | Nitrosamines Testing | LCMS/MS |
| Prilocaine | Nitrosamines Testing | LCMS/MS |
| Granisetron Hydrochloride | Nitrosamines Testing | LCMS/MS |
| Granisetron Hydrochloride | Nitrosamines Testing | LCMS/MS |
| Diphenhydramine hydrochloride | Nitrosamines Testing | LCMS/MS |
| Folic acid Hydrate | Nitrosamines Testing | LCMS/MS |
| Diphenhydramine hydrochloride | Nitrosamines Testing | LCMS/MS |
| Mefenamic acid | Nitrosamines Testing | LCMS/MS |
| Olanzapine | Nitrosamines Testing | LCMS/MS |
| Tramadol HCL | Nitrosamines Testing | LCMS/MS |
| Nitrofurantoin monohydrate USP | Nitrosamines Testing | LCMS/MS |
| Montelukast Sodium | Nitrosamines Testing | LCMS/MS |
| Tamsulosin Hydrochloride | Nitrosamines Testing | LCMS/MS |
| Midodrine Hydrochloride Tablet 2.5 MG & 5 MG | Nitrosamines Testing | LCMS/MS |
| Glycopyrrolate | Nitrosamines Testing | LCMS/MS |
| Silodosin | Nitrosamines Testing | LCMS/MS |
| Dronedarone HCL | Nitrosamines Testing | LCMS/MS |
| RS AMP | Related Substance | HPLC |
| Assay of AMP | Assay | HPLC |
| Rivaroxaban | Assay | HPLC |
| Cimetidine API | Related Substance | HPLC |
| Cimetidine Tablet | Related Substance | HPLC |
| Terbinafine | Related Substance | HPLC |
| Chenodeoxycholic acid | Assay | HPLC |
| Chenodeoxycholic acid | Related Substance | HPLC |
| 7-Keto Lithocholic acid | Assay | HPLC |
| 7-Keto Lithocholic acid | Related Substance | HPLC |
| Ursodeoxycholic acid | Assay | HPLC |
| Ursodeoxycholic acid | Related Substance | HPLC |
| Terbinafine HCL | Organochlorine Testing | GC-HS |
| Tamsulosin Hydrochloride | Nitrosamines Testing | GCMS/MS |
| Glycopyrrolate | Nitrosamines Testing | GCMS/MS |
| Silodosin | Nitrosamines Testing | GCMS/MS |
| Domeperidone | Cd,pb,As,Hg,Co,V,Ni,Mo,Cr,Fe | ICP/MS |
| Furosemide | Cd,pb,As,Hg,Co,V,Ni,Mo,Cr,Fe,Sb, Li,Cu | ICP/MS |
| Warfarin Sodium clathrate injection | Cd,pb,As,Hg,Co,V,Ni,Mo,Cr,Fe,Sb, Li,Cu | ICP/MS |
| Warfarin Sodium clathrate oral injection | Cd,pb,As,Hg,Co,V,Ni,Mo,Cr,Fe,Sb, Li,Cu | ICP/MS |
| Lanthanum Carbonate | Lanthanum ICP/MS |
